As diagnostic technologies continue to advance, it is becoming increasingly crucial to ensure that blood samples are collected and stored in a manner that preserves their integrity. The use of circulating cell-free DNA (cfDNA) as a biomarker for various diseases has led to the development of specialized cfDNA collection tubes that help improve pre-analytical sample quality. In this article, we will explore how cfDNA tubes enhance sample stability and reduce the risk of degradation, ultimately improving the reliability and accuracy of diagnostic testing results.
The Science Behind Sample Degradation: Factors Affecting Pre-Analytical Sample Quality
The science behind sample degradation is a complex and multifactorial process that can occur at any stage of the pre-analytical phase, from collection to processing. Factors such as temperature fluctuations, hemolysis, clotting, and improper storage conditions can all lead to changes in the properties of biological samples, affecting the quality and accuracy of diagnostic testing results.
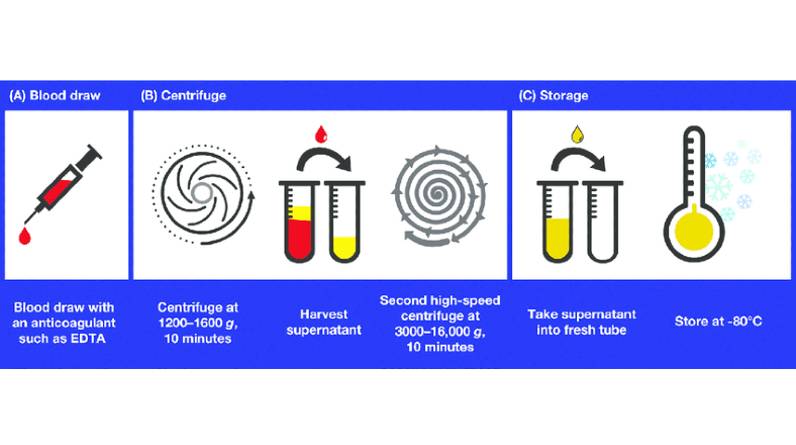
One area where sample degradation has been particularly challenging is in the use of circulating cell-free DNA (cfDNA) as a biomarker for disease detection. The fragile nature of cfDNA makes it especially susceptible to degradation during handling and storage. To address this issue, specialized cfDNA tubes have been developed that utilize stabilizing agents to improve sample stability and reduce the risk of degradation.
By understanding the factors that affect pre-analytical sample quality and utilizing advanced techniques such as this specialized cfDNA tube, researchers and clinicians can ensure optimal preservation of biological samples for accurate diagnostic testing results.
Benefits of Using cfDNA Tubes: Improving Sample Stability and Reducing Risk of Degradation
The benefits of using cfDNA tubes in blood sample collection are twofold: improving sample stability and reducing the risk of degradation. Regular blood collection procedures can expose DNA to various factors that can damage it, leading to inaccurate diagnostic testing results. CfDNA tubes protect against this outcome by containing a stabilization reagent that prevents cell death, preserving DNA integrity for up to 14 days at room temperature. This prolonged shelf life means there is less urgency in handling samples immediately after drawing them, allowing laboratories more flexibility when dealing with delayed lab processing times or unexpected shipping delays.
Another advantage of using these specialized tubes is reducing the likelihood of hemolysis, which contaminates specimens and yields unreliable test results. Small fragments such as those found in cfDNA require protection from physical stresses like shaking or centrifugation to prevent leaking out from broken cells due to hemolysis during extraction or storage. The added resistance provided by cfDNA tubes ensures that delicate biomolecules remain intact until they reach their destination facility for laboratory analysis; this reduces false positives and unnecessary diagnostic procedures while increasing accuracy across multiple medical specialties where sensitive analyses involving small amounts of material are necessary, including non-invasive prenatal testing (NIPT) and cancer screening tests.
See more about – QWORDLE
Implementing Best Practices: Ensuring Proper Handling and Storage of cfDNA Samples
Implementing best practices for the handling and storage of cfDNA samples is critical in preventing degradation, preserving sample integrity, and ensuring the reliability of diagnostic testing results. Proper collection techniques and tube selection are crucial to maintain optimal stability during pre-analytical processes. The use of specialized cfDNA tubes can help prevent hemolysis, ensure efficient separation from plasma components, and protect against contamination.
In addition to selecting appropriate collection tubes, it is important to store cfDNA samples at the appropriate temperature and duration. Refrigeration or freezing may be necessary depending on the intended analysis or storage timeframe. Proper labeling practices should also be implemented to minimize errors during sample identification. By following these best practices consistently and diligently throughout the pre-analytical phase, healthcare providers can optimize sample quality for robust downstream analysis that ultimately supports better patient outcomes.